BioCAT is a mature user facility that supports several types of experiments. One measure of the facility's scientific impact is the number and quality of the publications produced. As of June 2024, the facility and its users had published more than 700 articles, which had been cited more than 48,000 times with an average number of citations per article around 68.
A list of all BioCAT publications can be found in the APS database
When you publish your science from BioCAT, please follow the guidelines.
Workshops
BioCAT organizes occasional topic focused science workshops (in contrast to our more general training workshops). Videos from lectures at these workshop are listed below
MuscleX 3: Sarcomeric regulation mechanisms in health and disease
- Welcome and introduction to scientific missions at BioCAT by Thomas Irving
- Thick filament regulation in porcine myocardium by Weikang Ma
- The structurally-defined OFF and ON state can be decoupled with biochemically-defined super-relaxed and disordered-relaxed state by Saffie Mohran
- Single-molecule imaging of thick filament regulation in myofibrils by Neil Kad
- In situ structures from relaxed cardiac myofibrils reveal the organization of the muscle thick filament by Davide Tamborrini
- Titin activates myosin filaments in skeletal muscle by switching from an extensible spring to a mechanical rectifier by Caterina Squarci
- Cardiac Troponin T N-domain variant destabilizes the actin interface resulting in disturbed myofilament function by Maicon Landim-Vieira
- Minding the Gap: Myosin Binding Protein C steps over the Interfilament Space by Samantha Harris
- Right Ventricular Cardiomyocyte Sarcomere Dysfunction and Deficient Thick Filament Activation in Human Heart Failure with Right Ventricular Dysfunction by Vivek Jani
- Myosin activator Danicamtiv increases myosin recruitment and alters the chemomechanical cross bridge cycle in cardiac muscle by Farid Moussavi-Harami
Science Highlights
Below is a gallery of some of BioCAT's science highlights.
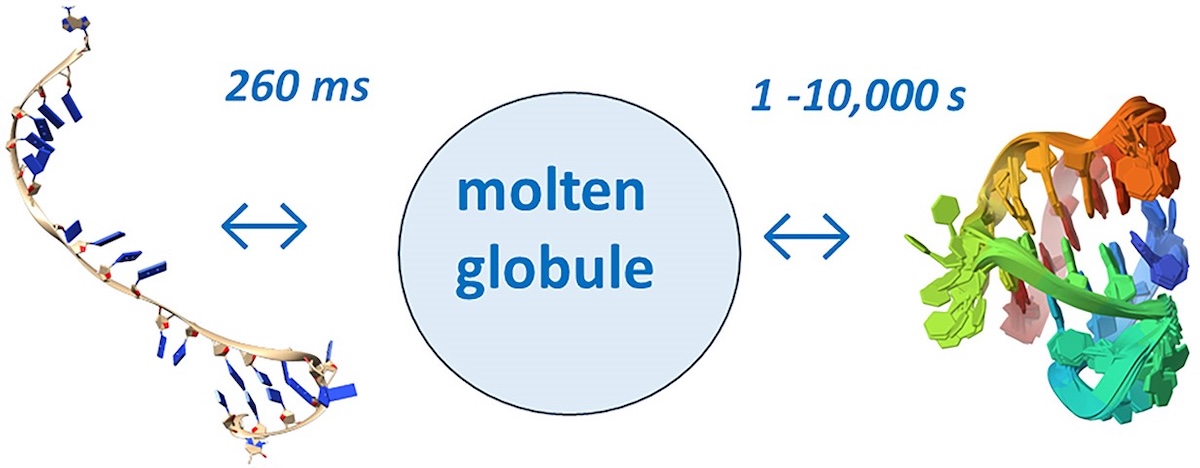
Unraveling folding pathways of dynamic DNA quadruplexes
G-quadruplexes (G4s) are non-B form DNA structures containing genomic regions rich in guanine that sometimes fold into and often act as transcriptional regulators. They are frequently located at regulatory sites including promoters, replication origins and telomeres11. Understanding their folding pathways, while important to a fundamental understanding of form and function, has been challenging. G4 quadruplex folding pathways are distinct from not only proteins but also duplex RNA and DNA12. Previous work characterizing folding pathways using FRET, CD or stopped-flow absorbance suggested a complex, multi-step pathway. Researchers from the University of Louisville, some of whom are frequent BioCAT users, performed continuous-flow mixing time-resolved SAXS experiments to directly structurally characterize, for the first time, early steps in the G4 quadruplex folding pathway.
Learn More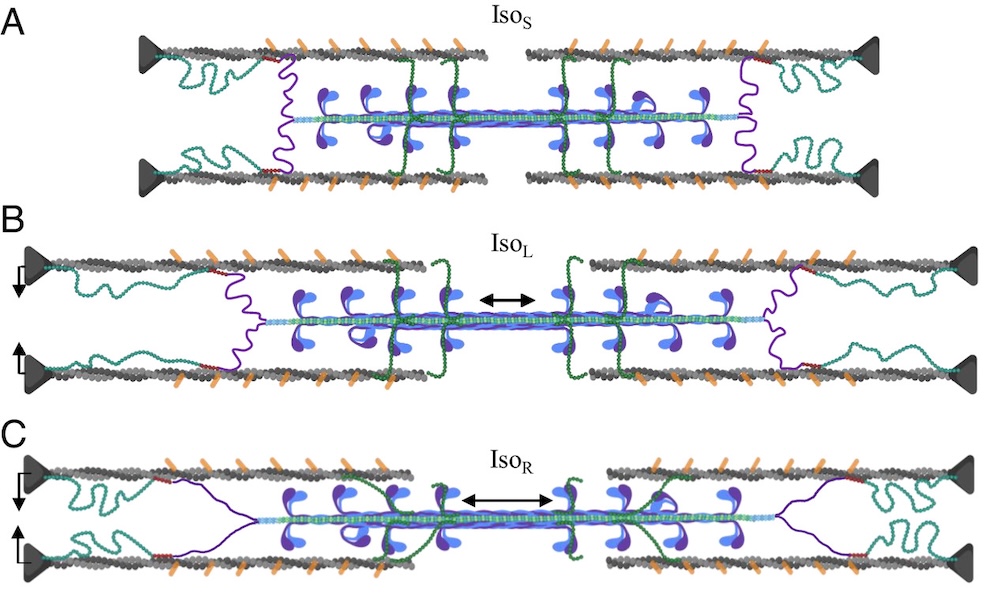
A role for titin in residual force enhancement in skeletal muscle
Residual force enhancement (RFE) is a property of skeletal muscle where more force is produced after an active stretch than if it were simply activated at the longer length. This property is important for jumping, locomotive, and stabilizing movements of the body. The molecular mechanism underlying RFE is not well understood. In muscle, the protein titin connects myofilaments and has been shown to have many roles in sarcomere stability and modulating contraction. The authors of this study investigated titin's function during contraction using small-angle X-ray diffraction of untreated WT mouse skeletal muscle and muscle where 50% of the titin was cleaved. They compared their results to those obtained from mdm titin mutant mce that do not show residual force enhancement.
Learn More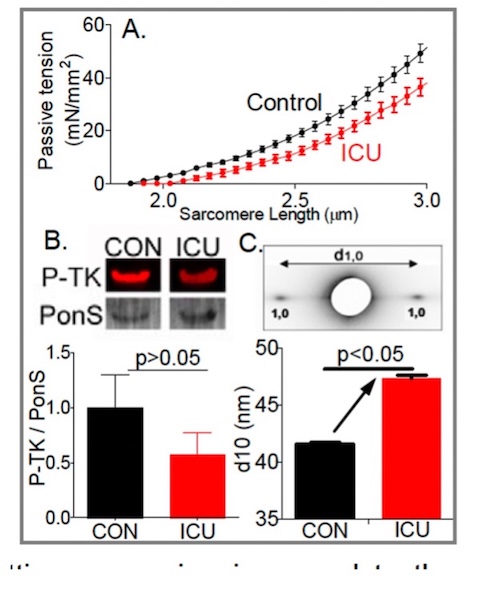
Role of titin in the pathophysiology of diaphragm weakness during mechanical ventilation
Diaphragm unloading during mechanical ventilation is an important clinical problem. The diaphragm is the main muscle of respiration and contracts during each breath, thereby changing the anatomic configurations of the chest wall so that air flows into the lungs. Increased diaphragm loading is associated with diaphragm fiber contractile dysfunction, atrophy and injury. Whereas these effects of increased loading on the diaphragm take months or years to develop, the effects of decreased loading, as occurs when ICU patients are mechanically ventilated, occur extremely rapidly, within hours. Not known is which structures sense the mechanical unloading of the diaphragm and set in motion the molecular cascades leading to atrophy. Here we test the hypothesis that the mechano-sensor protein is titin, a giant elastic protein connecting Z-disks and the thick filaments in the A-band.
Learn More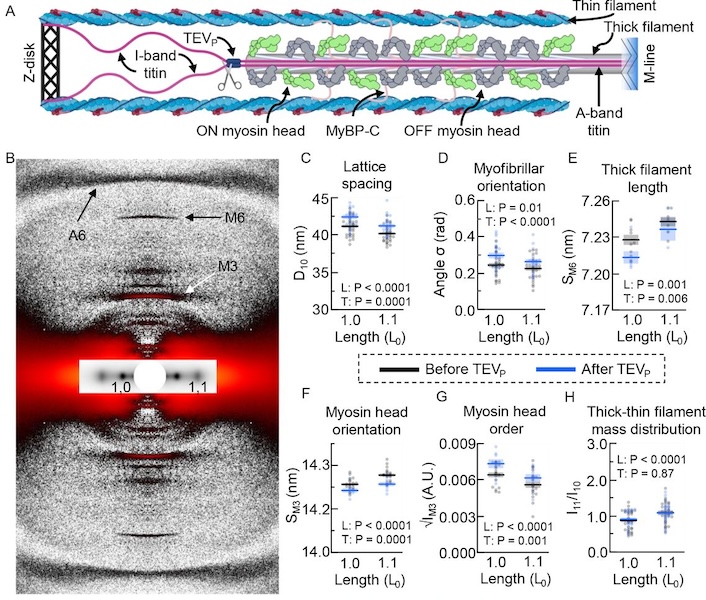
Titin-Based Force Modulates Cardiac Thick and Thin Filaments
The Frank-Starling Law of the Heart states that the heart’s stroke volume increases with greater preload due to increased venous return, allowing the heart to adapt to varying circulatory demands. At the molecular level, increasing preload increases sarcomere length (SL), which alters structures w ithin the sarcomere that are correlated to increased calcium sensitivity upon activation. The titin protein, spanning the half-sarcomere acts as a spring in the I-band, applies a SL-dependent passive force on the myosin containing thick filaments changing its structure and functional properties. Altered titin-based forces play a crucial role in the etiology of many cardiomyopathies; however, the disease state obscures titin’s role, impeding therapeutic solutions. The authors studied titin’s specific role and concluded that reducing titin-based forces blunts structural changes in both thick and thin filaments while leaving the length-dependent OFF-to-ON transition mechanism intact, indicating a clear role for titin in the Frank-Starling mechanism.
Learn More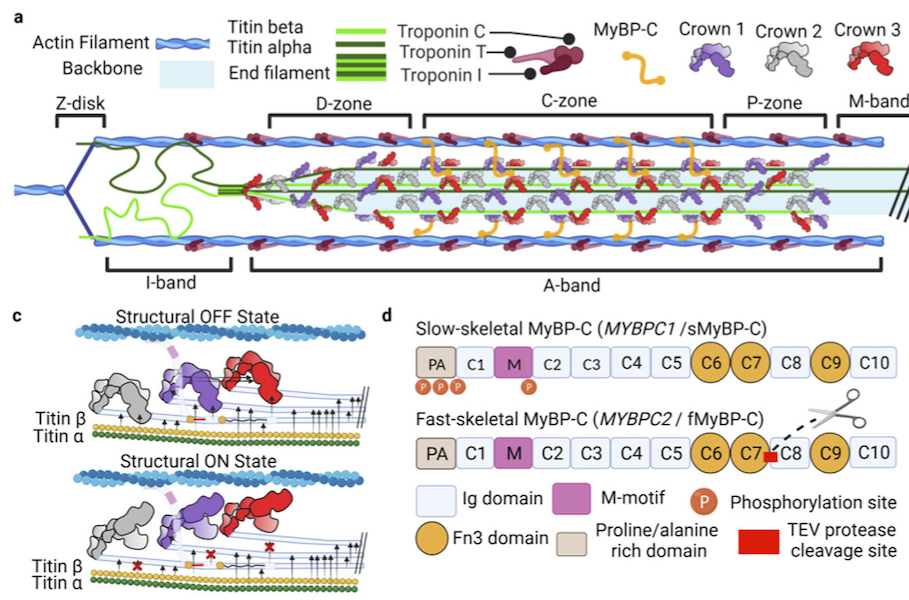
Myosin-binding protein C regulates the sarcomere lattice and stabilizes the OFF states of myosin heads
Muscle contraction is produced via the interaction of myofilaments and is regulated so that muscle performance matches demand. Myosin-binding protein C (MyBP-C) is a long and flexible protein that is thought to control muscle contraction via the regulation of myosin motors, as mutations lead to debilitating disease. Here the authors used combination of mechanics and small-angle X-ray diffraction to study the effects of immediate and selective removal of the particular domains of fast MyBP-C on sarcomere structure and function in permeabilized skeletal muscle. They concluded that the MyBP-C domains play an important role in contractile performance.
Learn More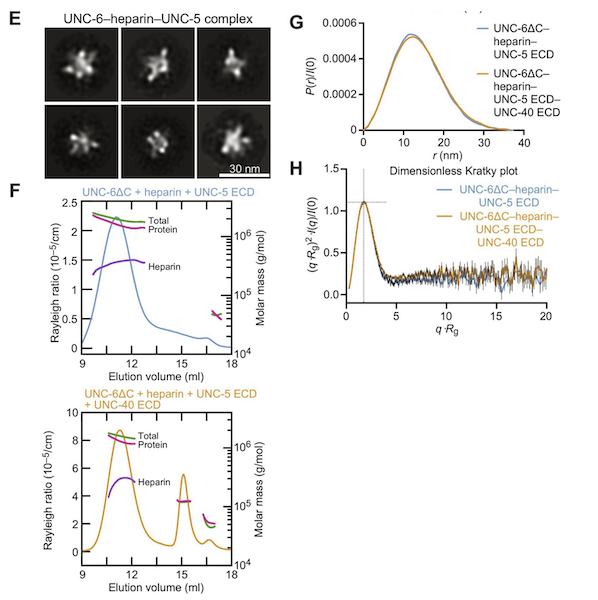
Structural insights into the formation of repulsive netrin guidance complexes
Netrins are a conserved class of proteins involved in synaptic connectivity of the nervous system in bilaterian animals. They act as secreted guidance cues, with the unique ability to exert repulsive and attractive responses on growing axons. They are also known to be involved in cell proliferation, migration and differentiation, and are therefore targets for treating cancer and insulin resistance. During axon growth and cell migration, the presence of the receptor Uncoordinated-5 (UNC-5) on target cells results in repulsion. However, the exact mechanism involved in the induction of repulsive forces has been relatively unknown due to the lack of biochemical and structural information about these systems. Researchers at the University of Chicago and Stanford University, in collaboration with BioCAT staff, showed that UNC-5 is a heparin-binding protein, determined its structure bound to a heparin fragment, and could modulate UNC-5–heparin affinity using a directed evolution platform or structure-based rational design.
Learn More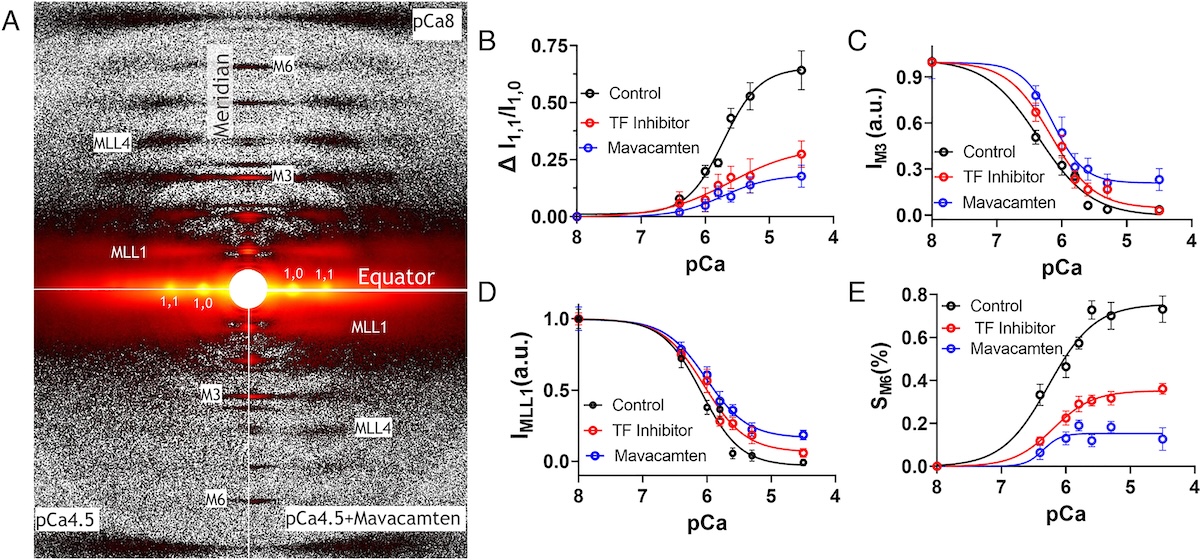
Myosin in autoinhibited off state(s), stabilized by mavacamten, can be recruited in response to inotropic interventions
Mavacamten is the first myosin-targeted small-molecule therapy approved by the Food and Drug Administration to treat obstructive hypertrophic cardiomyopathy by attenuating excessive myocardial sarcomere activity. Mavacamten regulates cardiac function at the sarcomere level by selectively but reversibly inhibiting the enzymatic activity of myosin. It shifts myosin toward ordered off states close to the thick filament backbone making them unavailable for binding to actin and generating force. It is necessary, however for the heart to adjust its output to ensure sufficient cardiac output, especially during increased physiological demands. It was unknown whether mavacamten stabilized heads could still be recruited by the usual physiological inotropic mechanisms for the patient to be able to adapt to changing demands on their hearts. The authors of this study provided direct evidence the mavacamten-promoted off states of myosin in the thick filament are at least partially activable, thus preserving cardiac reserve mechanisms.
Learn More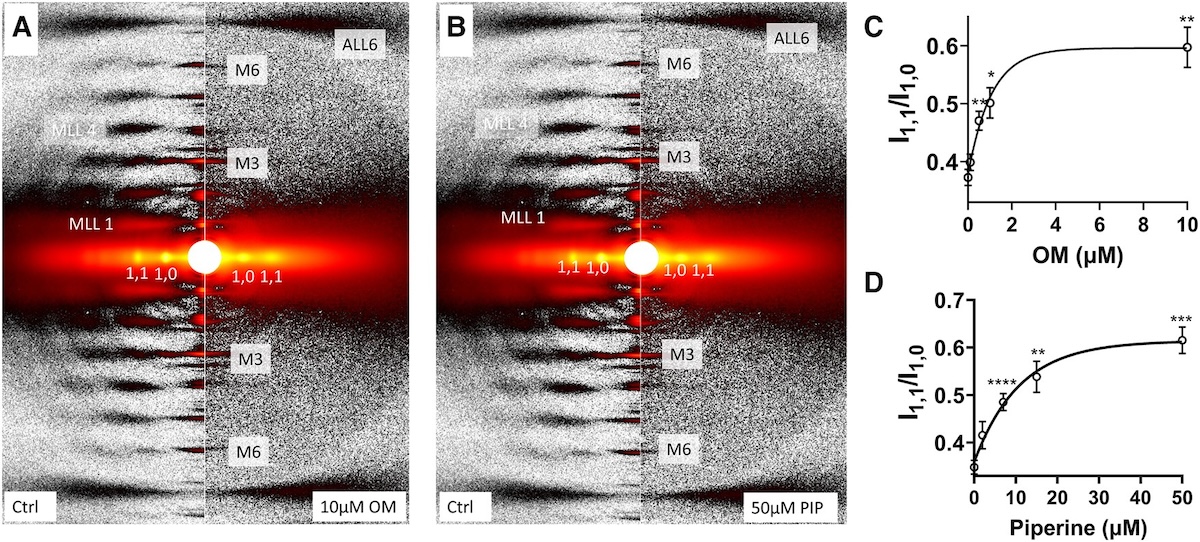
The structural OFF and ON states of myosin can be decoupled from the biochemical super- and disordered-relaxed states
Myosin-based thick-filament regulation is now known to be critical for muscle contraction with myosin dysregulation found in hypertrophic and dilated cardiomyopathies but many details of thick filament regulation remain to be discovered. Myosin ATPase assays have demonstrated that under relaxed conditions, myosin may reside either in a high-energy-consuming disordered-relaxed (DRX) state available for binding actin to generate force or in an energy-sparing super-relaxed (SRX) state unavailable for actin binding. X-ray diffraction studies have shown that the majority of myosin heads are in a quasi-helically ordered OFF state in a resting muscle and that this helical ordering is lost when myosin heads are turned ON for contraction. It has been assumed that myosin heads in SRX and DRX states are equivalent to the OFF and ON states. Our results show that biochemically defined SRX and DRX can be decoupled from structurally defined OFF and ON states.
Learn More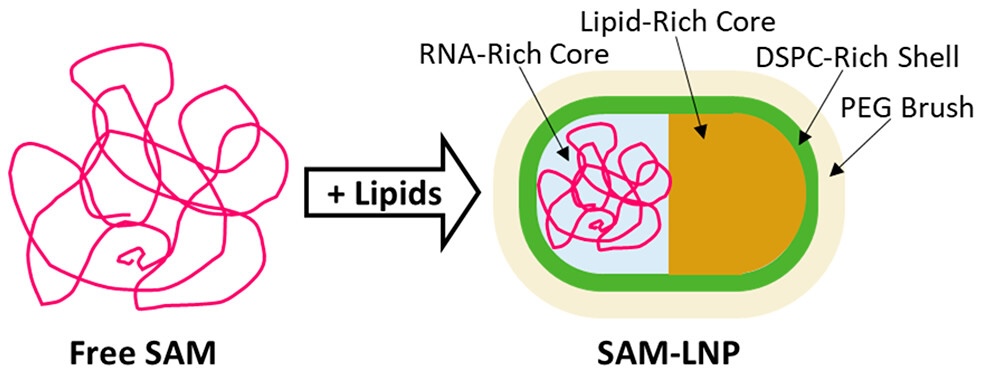
Insights Into Unusual Morphologies of Lipid Nanoparticle Encapsulations
The success of mRNA-based COVID-19 vaccines has brought increased attention to lipid nanoparticles (LNPs) as an RNA therapeutic delivery method. One of the main challenges of LNPs as a delivery method remains the significant percentage of unloaded LNPs in most (if not all) RNA-LNP formulations, highlighting a need for better characterization of their formation, composition and morphology. While there have been a number of recent advances in understanding the formation and structure of LNPs, studies exploring the influence of non-mRNA RNAs on LNP morphology remain limited. Of particular interest is the delivery of self-amplifying mRNA (SAM), which has the potential to both significantly lower dosage as well extend the antigen expression lifetime in the body. However, given that SAM molecules are typically significantly larger than conventional nonreplicating mRNA, and that larger nucleic acids are known to promote the formation of significant populations of empty LNPs, further work is needed to better understand the formation, composition and morphology of LNPs in order to better inform LNPs as a delivery system for promising therapeutic strategies that rely on larger nucleic acids.
Learn More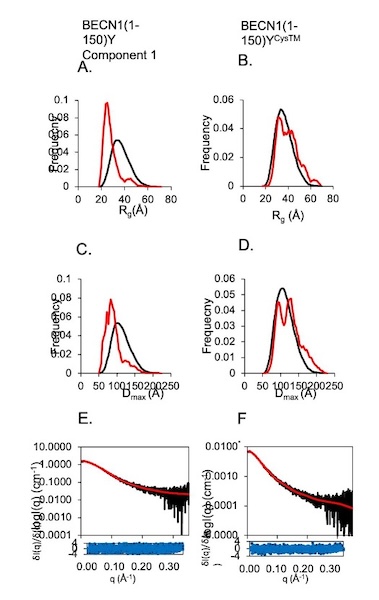
Invariant BECN1 CXXC Motifs Bind Zn2+ and Regulate Structure and Function of the BECN1 Intrinsically Disordered Region
Autophagy is a conserved lysosomal degradation pathway that degrades un-needed cellular components such as misfolded, aggregated, mutated and damaged proteins, organelles, and pathogens. Autophagy dysfunction is implicated in numerous diseases including neurodegenerative disorders, muscular diseases, cardiomyopathy, cancer and infectious diseases. Many proteins involved in autophagy contain intrinsically disordered regions (IDRs) that do not form stable secondary or tertiary structure. The structural flexibility of IDRs is thought to enable diverse and multiple interactions enabling them to regulate cell signaling pathways. Many IDRs have been shown to fold upon binding to ligands. BECN1, a key autophagy protein involved in autophagosome nucleation, contains two invariant CxxC motifs within a large BECN1 intrinsically disordered region (IDR) at the BECN1 N-terminus. The goal of the research was to uncover the functional roles of the invariant CxxC motifs which were hitherto not understood.
Learn More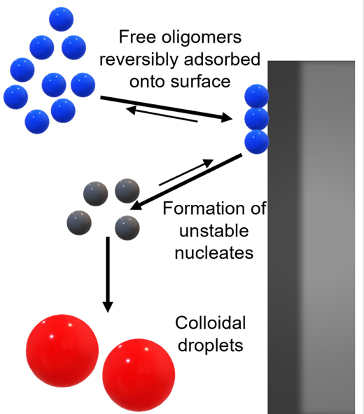
Surface-mediated spontaneous emulsification of the acylated peptide semaglutide
Semaglutide (SMG) is class of modified, acetylated peptide mimic commonly used as a commercial therapeutic to treat type-2 diabetes and obesity. Like other classes of peptide mimic therapeutics, SMG’s suffer from physical instabilities, including various aggregation and degradation pathways but also spontaneous emulsification into colloidal structures in the presence of certain hydrophobic surfaces, a process often termed “ouzo formation.” Researchers at the University of Delaware Center for Neutron Science, in collaboration with Eli Lilly, used a variety of biophysical methods including small-angle X-ray scattering (SAXS), circular dichroism (CD) and dynamic light scattering (DLS) to elucidate the fundamental physical mechanisms behind ouzo formation. This work provides a foundation for predicting ouzo-like formation in related molecules, which may help guide future formulations and storage methods for a range of therapeutics.
Learn More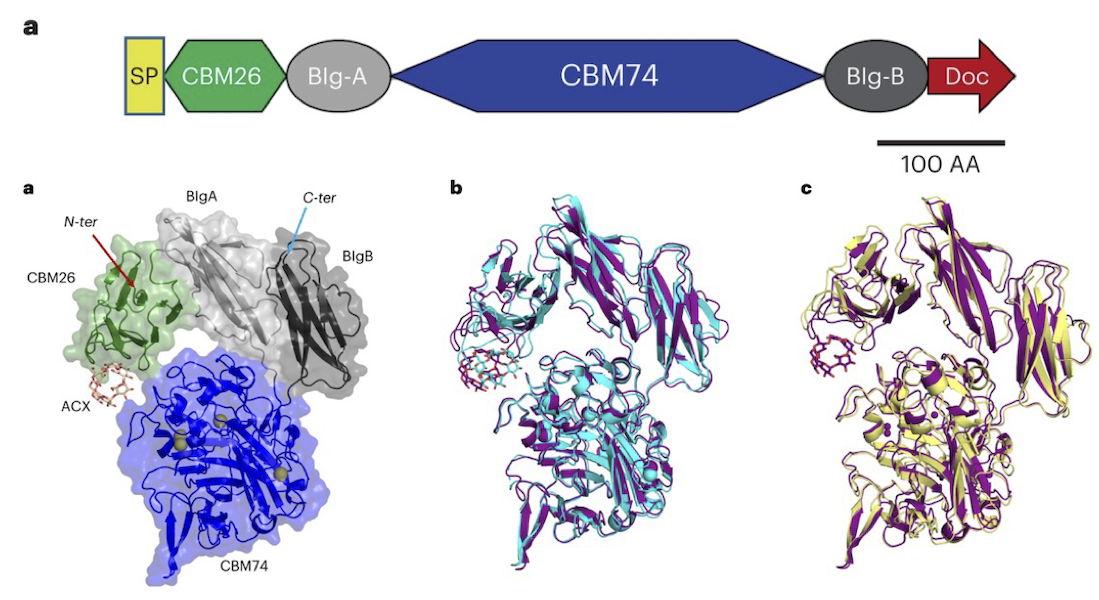
Characterization of starch-degrading enzymes
The ways in which starches, in particular digestion-resistant starches, are accommodated by gut bacteria remains relatively poorly understood at the molecular level. Digestion-resistant starches are accessed by specialized gut bacteria with specific carbohydrate-binding systems. The authors present a structural and functional characterization by crystallography, SAXS, native mass spec and other methods of one such system from Ruminococcus bromii (Sas6). Together, these data allowed the authors to elucidate the starch granule recognition mechanism, providing foundational work for both engineering of starch-degrading systems as well as gut-microbiome focused health applications.
Learn More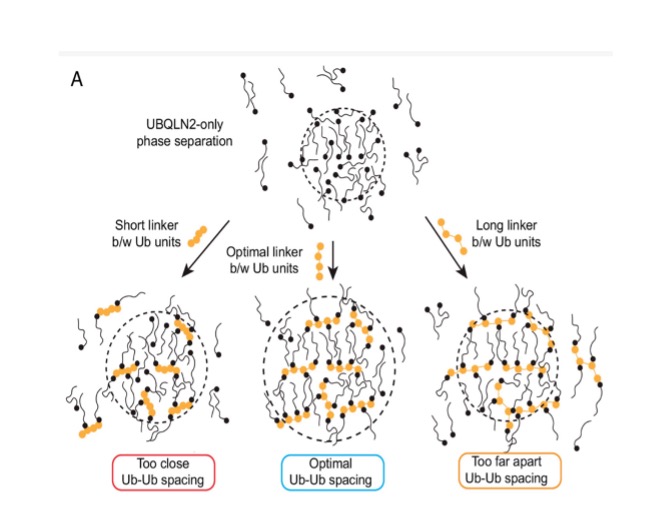
Polyubiquitin ligand-induced phase transitions are optimized by spacing between ubiquitin units
Biomolecular condensates are involved in a range of cellular processes including stress response, protein degradation and gene expression. These condensates contain a wide range of unique macromolecules, but the drivers of this condensation, referred to as scaffolds, comprise only a very small fraction. The non-driver components are commonly referred to as ligands and may not phase separate on their own but nonetheless may help regulate assembly, disassembly and other material properties. One such ligand is ubiquitin (Ub) or its linked multimers (polyubiquitin chains), which are attached as posttranslational modifications to partner proteins and help determine various downstream signaling outcomes such as DNA repair. There is growing evidence that suggests the involvement of polyUb chains in phase separation acts as a mechanism for the reading and interpretation of said Ub code in the cell. Research from the Castaneda lab at Syracuse University has worked towards understanding the molecular rules by which polyUb chains are able to regulate biomolecular condensation.
Learn More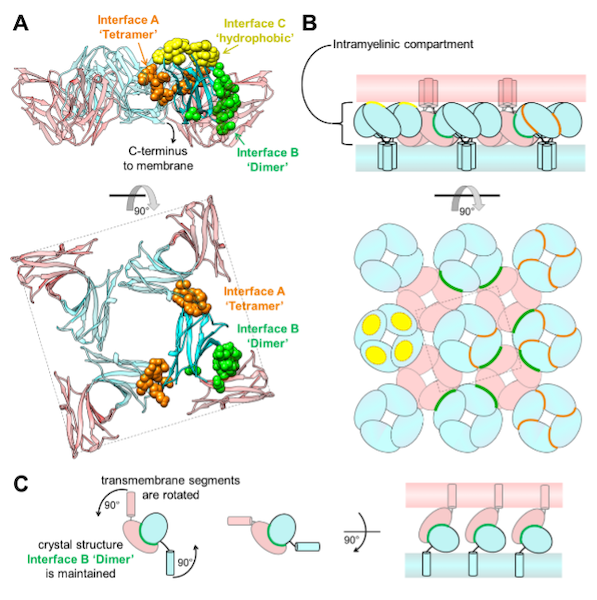
Homomeric interactions of the MPZ Ig domain and their relation to Charcot-Marie-Tooth disease
Charcot Marie Tooth (CMT) disease is the most common form of heritable peripheral neuropathy, which are a group of inherited diseases affecting the peripheral nervous system (PNS). Myelin protein zero (MPZ) is necessary for normal myelin structural and function comprises ~50% of all proteins in the PNS; mutations in MPZ account for around 5% of CMT cases. The authors performed nuclear magnetic resonance spectroscopy and small angle X-ray scattering (SAXS) analysis on a region of this protein. They were able to correlate different types of Charcot-Marie-Tooth disease symptoms to subregions within this protein.
Learn More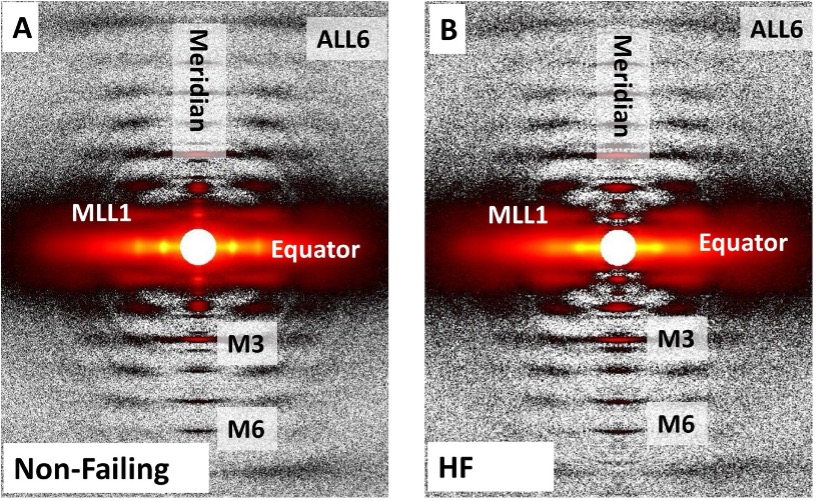
Right Ventricular Sarcomere Contractile Depression and the Role of Thick Filament Activation in Human Heart Failure With Pulmonary Hypertension
Right ventricular (RV) contractile dysfunction commonly occurs and worsens outcomes in patients with heart failure with reduced ejection fraction and pulmonary hypertension (HFrEF-PH). However, such dysfunction often goes undetected by standard clinical RV indices, raising concerns that they may not reflect aspects of underlying myocyte dysfunction. To address the need for better diagnostics, the authors sought to characterize RV myocyte contractile depression in HFrEF-PH, identify those components reflected by clinical RV indices, and uncover underlying biophysical mechanisms.
Learn More
Proteins in Heart Muscle Can Produce More Oomph than Previously Thought
Recent research by a team of investigators from the Illinois Institute of Technology and the University of Washington presents a more detailed description of the positional changes of the myosin proteins within the heart as they prepare for contraction, and demonstrates how the myosin’s behavior directly affects the amount of force created during muscle contraction, revealing new focus points for medicines.
Learn More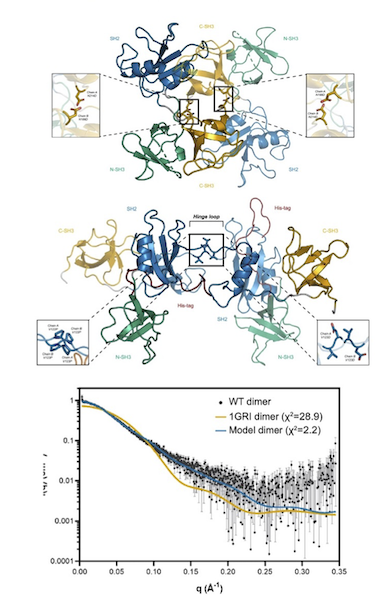
GRB2 dimerization mediated by SH2 domain-swapping is critical for T cell signaling and cytokine production
Adaptor proteins are accessories to main proteins in signal transduction pathways that usually lack intrinsic enzymatic activity but instead facilitate the linking of binding partners together to enable the formation of larger signaling complexes. One widely expressed adaptor protein is the growth factor receptor-bound protein 2 (GRB2), which facilitates formation of cytoplasmic signaling complexes from a wide array of binding partners. As a consequence, the structure and function of GRB2 have become major areas of investigation for novel areas of interventions against various human diseases. Here, the authors showed that a novel dimeric GRB2 conformation with domain-swapping between SH2 domains and monomer/dimer transitions was critical for GRB2 to facilitate early signaling complexes in human T cells.
Learn More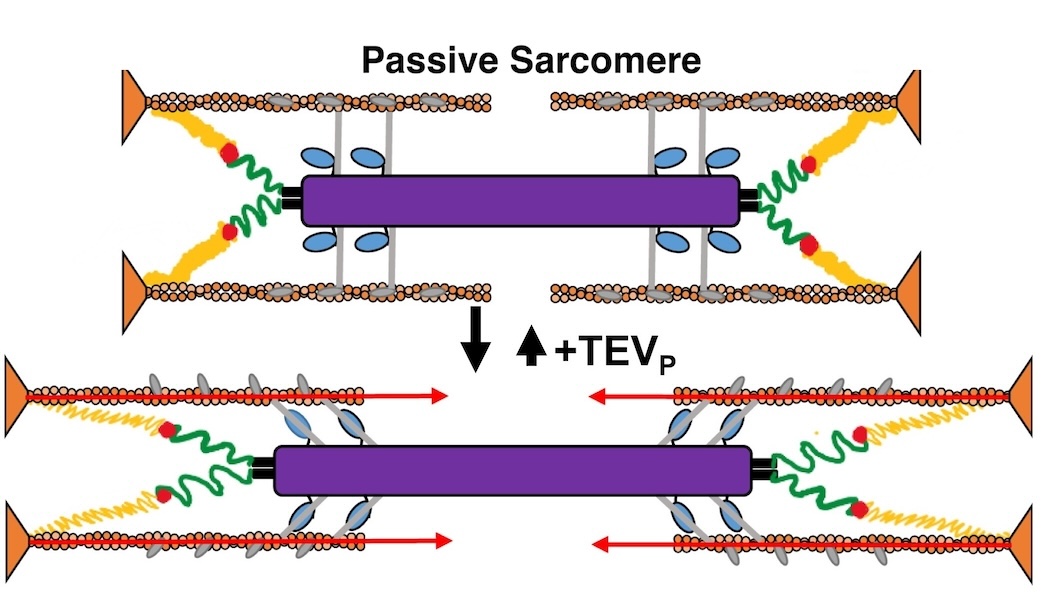
Titin force in muscle cells alters lattice order, thick and thin filament protein connections
Muscles can produce more force when stretched to a longer length at the same level of activating calcium, a poorly understood phenomenon known as myofilament length dependent activation (LDA). It was suggested several years ago that passive force generated by the giant elastic protein titin could be the length sensor behind this phenomenon, but direct evidence has been lacking. These experiments firmly established titin as the length sensor in LDA and showed that LDA involves structural changes in both thick and thin filaments.
Learn More
Understanding Phase Separation Could Impact Treatment of Neurodegenerative Disease
Living cells are amazing little biochemical factories that conduct countless chemical reactions in a cellular soup packed with lipids, proteins, nucleic acids, and ions, keeping them all in their proper places at any given time. Cells maintain this organization even while carrying out complex tasks such as cell division, signaling, transcriptional regulation, and stress responses. One example of this is the careful management of stress granule formation, a process in which membraneless organelles transiently form to control the utilization of mRNA during stress. These granules form and disperse through reversible liquid-liquid phase transitions involving proteins and RNA in the granules. Recent research has demonstrated that RNA-binding proteins in these granules contain intrinsically disordered sequences, called prion-like low-complexity domains (PLCDs), that are critical to regulation of these reversible phase transitions. There is also mounting evidence that these transitions may be disrupted in neurodegenerative diseases, like amyotrophic lateral sclerosis (ALS), in which mutations in PLCD-containing proteins, such as hnRNPA1, have been implicated as a cause of the disease. Recent work that relied on data gathered at the U.S. Department of Energy’s Advanced Photon Source (APS), an Office of Science user facility at Argonne National Laboratory, and published in the journal Nature Chemistry aimed to learn more about how these phase transitions are regulated. These findings will provide important information about the causes of diseases like ALS.
Learn More
Targeting Cancer at the Level of DNA Expression
The last 20 years have brought a revolution in targeted therapies for cancer. Small-molecule inhibitors and monoclonal antibodies that target a specific aberrant protein in tumors have provided cancer patients with treatments that are associated with fewer side effects and longer survival than conventional chemotherapy. This has been, in large part, the result of intensive research into the role of oncogenes in cancer development. Oncogenes are normal cellular genes that have become mutated in such a way that they aberrantly promote the uncontrolled cell growth seen in cancer. They are often proteins involved in growth control or activation of cellular signaling; inhibiting these mutated proteins has proven to be effective in stopping the growth of many cancers. Research by a team from the Brown Cancer Center at the University of Louisville in Kentucky using the U.S. Department of Energy’s Advanced Photon Source (APS) and published in the journal Nucleic Acids Research promises to extend these treatment possibilities to control these oncogenes at the gene expression level. The work, based on the discovery that DNA in the promoter region of many genes forms higher order structures that could provide unique druggable targets for intervention, extends structural knowledge of the promoter regions of three important oncogenes.
Learn More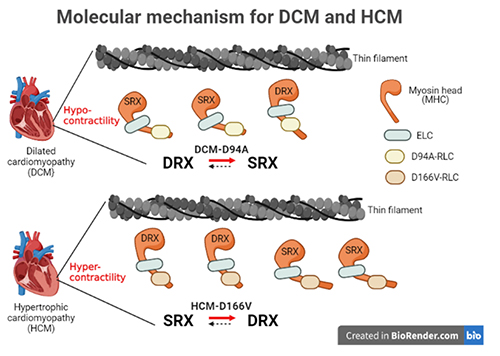
Understanding the Structural Implications of Genetic Mutations in Heart-Muscle Disease
Cardiomyopathies are diseases of the heart muscle in which the muscle of the pumping chamber (ventricle) can become enlarged (dilated cardiomyopathy; DCM) or thickened (hypertrophic cardiomyopathy; HCM), potentially leading to heart failure. There are currently no effective treatments but the disease often has a genetic component related to mutations in the heart muscle proteins that are involved in muscle contraction, so some researchers have focused their therapeutic development efforts on correcting these muscle contraction problems based on the structural basis of the defect. A recent study from a team of researchers using the U.S. Department of Energy’s Advanced Photon Source (APS) employed humanized mouse models expressing mutations observed in patients with HCM and DCM to evaluate the structure-function relationships and the changes observed in cardiac muscle contraction with these mutations. The work, published in the Proceedings of the National Academy of Sciences of the United States of America, provides a deeper understanding of the effects of cardiomyopathy-causing gene mutations on heart muscle contraction that could lead to the development of new therapies for this potentially life-threatening disease.
Learn More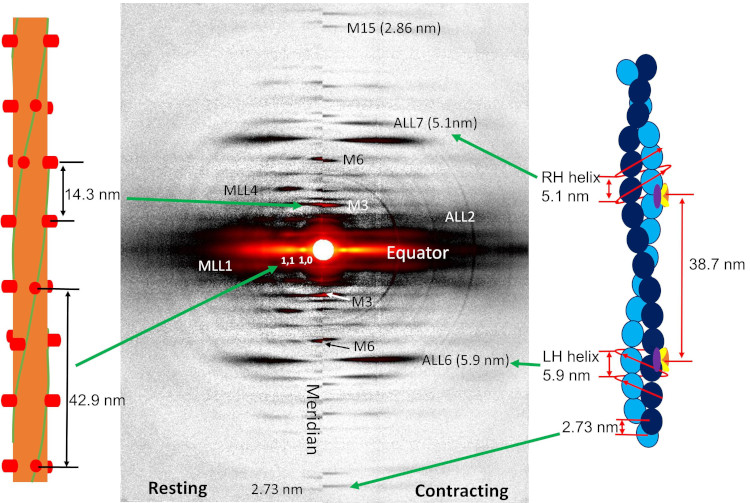
New Resource for the Muscle Diffraction Community
BioCAT staff have just published a review article, Ma & Irving, 2022 Int. J. Mol. Sci. 2022, 23(6), 3052, on the use of small angle X-ray fiber diffraction for studying skeletal and cardiac muscle disease. The article consists of a guided tour of the various diffraction features that can be used to extract specific pieces of information that can be used to provide insights into the structural basis of pathology. The article also contains a comprehensive review of the literature reporting diffraction studies of muscle that illustrates how small angle fiber diffraction has increased our understanding of specific muscle diseases such as hypertrophic cardiomyopathy, dilated cardiomyopathy, and nemaline myopathy.
Learn More
What Bacterial Pathogens Can Teach Us about Protein Folding
Protein folding is one of the fascinating unanswered questions in biology. How does an amino acid sequence that is unfolded when it leaves the ribosome manage to fold properly into a highly ordered, lightning-fast enzyme or sturdy structural protein? Why don’t all the proteins in the cell instead just stick to each other, aggregating into a big mess? A unique model system in bacteria may hold some of the answers to these questions. The system involves the study of what are termed autotransporter proteins. These proteins have a highly specialized protein folding process that attracted the attention of a team of researchers who have used this bacterial system as a model to determine what allows these unique proteins to maintain their disordered state in the periplasm. The work includes studies carried out at BioCAT. The authors believe their work will provide important information toward understanding basic questions of protein folding and tests long-held theories about how this remarkable biological process works.
Learn More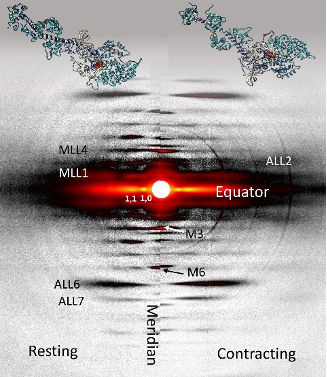
Relaxation at the Molecular Level
The molecular interactions between the proteins myosin and actin that generate force during muscle contraction are some of the most well-studied molecular interactions in biology. However, there are some congenital skeletal muscle disorders and types of heart failure where relaxation of the muscle, rather than the force generation part of the cycle, appears to be the problem, and there are currently no available treatments that affect relaxation specifically. Recent work conducted at BioCAT used a unique transgenic mouse model, time-resolved small-angle x-ray diffraction, and molecular dynamics simulations to discover more about how myosin and actin interact during skeletal muscle relaxation. This research may help identify new treatments for neuromuscular disorders associated with impaired muscle relaxation kinetics.
Learn More
Understanding the Physiology of the Human Heart through the Study of Tarantula Muscles
A research team has found an unlikely source of inspiration for understanding how the human heart works and how we might design better drugs for conditions like hypertrophic cardiomyopathy: tarantulas. The source of nightmares for arachnophobes and the household pets for arachnophiles are inspiring researchers to take new approaches to understanding diseases that alter how heart muscle cells contract and relax. But, before getting to the human heart, there is more to learn about the physiology of tarantula muscles. The researchers set out to understand how contractions in tarantula muscle cells are activated and why are muscle twitches that follow a sustained muscle contraction (post-tetanic) more forceful than those that don’t (pre-tetanic). Their results provide evidence that phosphorylation, the chemical addition of a phosphoryl group (PO3-) to an organic molecule, plays a key role in muscle activation and post-tetanic potentiation (PTP) in tarantula muscles.
Learn More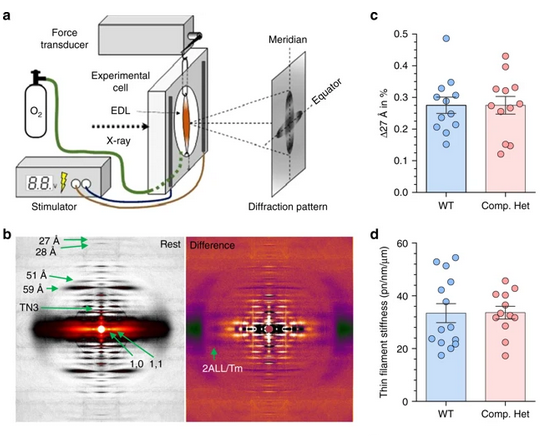
Key Insights into an Inherited Muscle Disease
The gene NEB encodes for the skeletal muscle protein nebulin. Mutations in NEB cause the disease nemaline myopathy, which is one of the more common inherited myopathies. Patients with this muscle disorder have muscle weakness in multiple different parts of their body and can also experience difficulties with feeding or breathing. Currently, there is no cure for nemaline myopathy and treatment options are limited. A team of researchers from the University of Arizona and BioCAT working to provide new insights into the pathogenesis of this skeletal muscle disorder, report a new mouse model of nemaline myopathy that exhibits similar symptoms to those identified in human patients. Importantly, the new mouse model of this disease can be used to test future therapeutics. Future studies are warranted to determine if interventions can relieve disease symptoms in these mice. If successful, such therapeutics could be used for improving the quality of life in human patients.
Learn More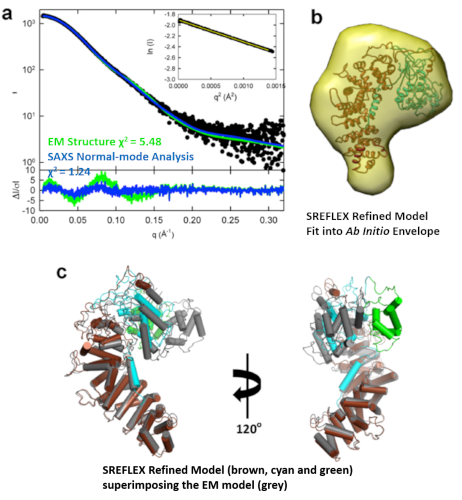
Structure-Function Studies Elucidate GPCR-Independent Regulation of G-proteins
Guanine nucleotide binding proteins popularly known as G-proteins, involved in a variety of cellular signal transduction pathways are heterotrimeric proteins consisting of α, β, and γ subunits. Ric8A is known to be both a chaperone for the assembly of the α-subunit of G-proteins, and a Guanine nucleotide Exchange Factor (GEF). McClelland et al., have conducted a detailed structural analysis on the complex between Ric8A and Gαi1 using cryoEM, X-ray crystallography, and SAXS.
Learn More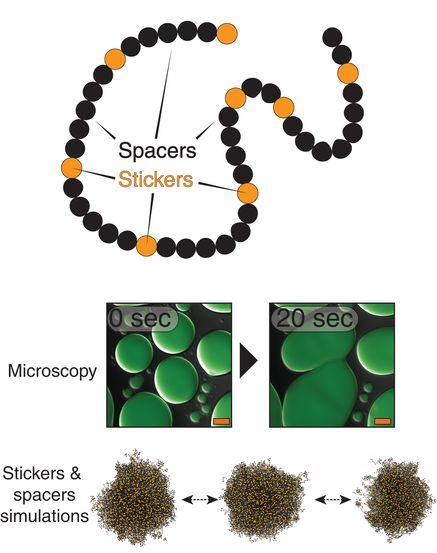
Uncovering Unique Structural Features in Protein Regions Associated with ALS
Prion-like domains (PLDs) have become a topic of interest because of their connection with a variety of debilitating brain diseases, such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia. In fact, mutations in PLDs of some genes have been shown to cause neurodegenerative disease. A recent study using data obtained at BioCAT completed a comprehensive biophysical investigation of PLDs in the protein hnRNPA1 to uncover the major behavioral and structural features of these domains. This meaningful work may lead to discoveries that can help individuals living with such neurodegenerative diseases.
Learn More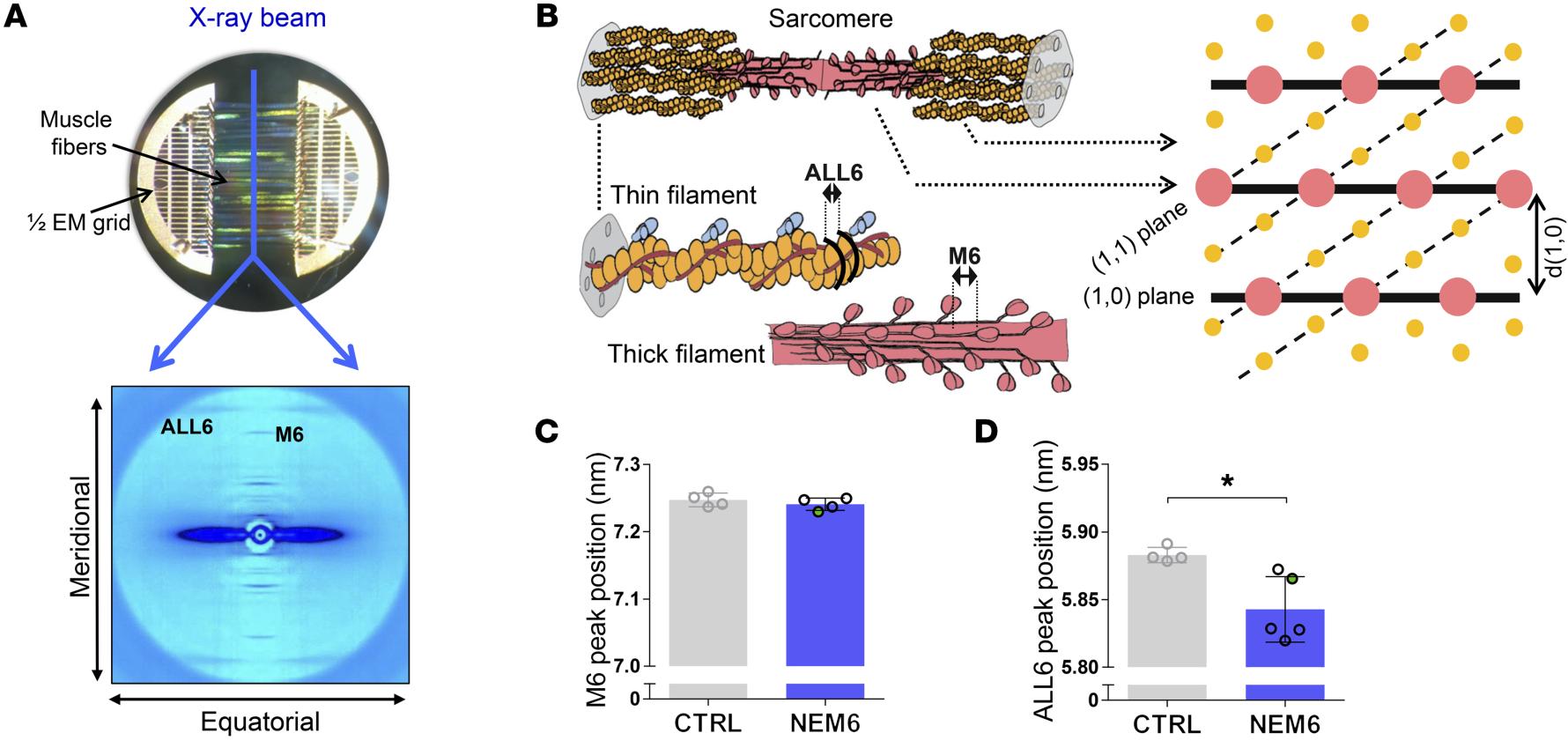
Sarcomere Structure and Nemaline Myopathy
Nemaline myopathy (NM) is one of the most common congenital non-dystrophic myopathies and is characterized by severe hypotonia, muscle weakness, feeding difficulties, respiratory failure, and the presence of nemaline bodies (rods) in skeletal muscle biopsies. One form of nemaline myopathy is caused by mutations in the KBTBD13 (NEM6) gene. A combination of transcranial magnetic stimulation-induced muscle relaxation, muscle fiber- and sarcomere-contractility assays, super-resolution microscopy, and low angle X-ray diffraction at BioCAT revealed that the impaired muscle relaxation kinetics in NEM6 patients are caused by structural changes in the thin filament, a sarcomeric microstructure.
Learn More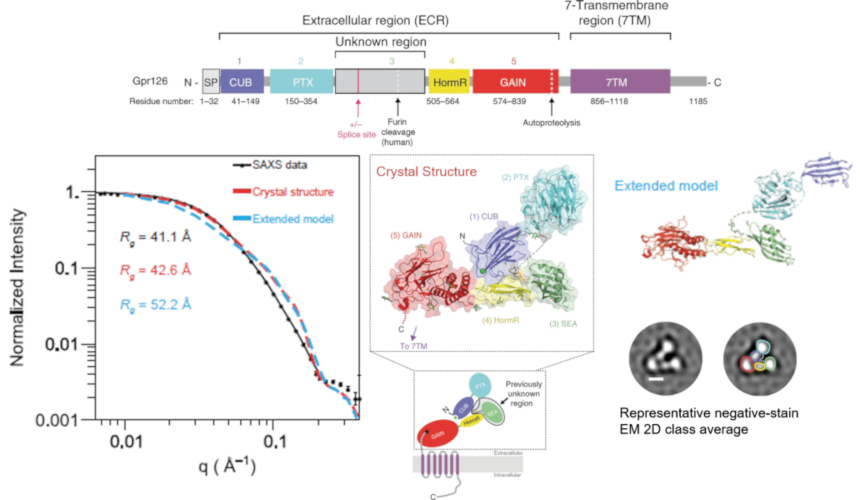
Structure-Function Understanding of aGPCR ECRs Critical for Drug-Design
Cellular communication mediated by a variety of cell-surface receptors involves ligand induced conformational changes in the extracellular region (ECR). A variety of drugs such as cetuximab (Epidermal Growth Factor Receptor), etrolizumab (Integrins), and erenumab (calcitonin receptor-like receptor) function by trapping ECRs in specific conformations and have proved to be effective therapeutic agents in several cancers, bowel diseases, and migraine. Leon et al., studied a class of relatively understudied G-protein couple receptors (GPCRs) called adhesion-GPCRs (aGPCRs) which have a structurally unique ECR with a diverse set of mechanistic possibilities.
Learn More
Cool Temperatures During Hibernation May Freeze Muscle Contraction to Save Energy
Striated muscle contraction is a highly regulated process that involves an orchestrated series of events within the muscle’s contractile units, which are also known as sarcomeres. In a recent study, researchers studied the effect of low temperature on mammalian skeletal muscle contraction. They found that cooler temperatures reduce force generation by trapping filaments in the muscle sarcomeres in a refractory state that cannot undergo contraction and utilize adenosine triphosphate (ATP). This mechanism provides important insight into how hibernating animals may conserve energy while still allowing vital functions in the body to continue.
Learn More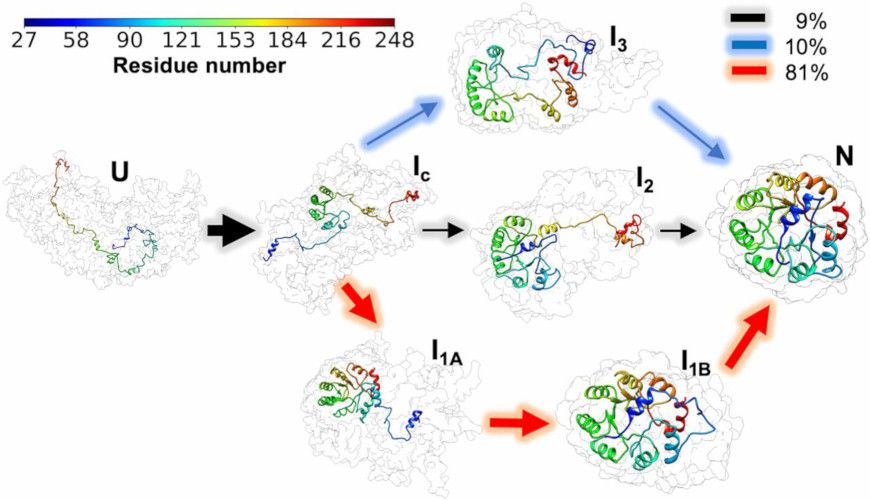
Frustration and Folding of a TIM Barrel Protein
In their continuing endeavor to understand misfolding proteins as part of the etiology of a variety of diseases, the Matthews lab particularly focuses on the different factors that impede a protein’s path from the unfolded state to the global free energy minimum. The complexity of the folding trajectory understandably depends on the size of the protein mostly because of the formation of intermediates many of which often stall the formation of an optimal native conformation.
Learn More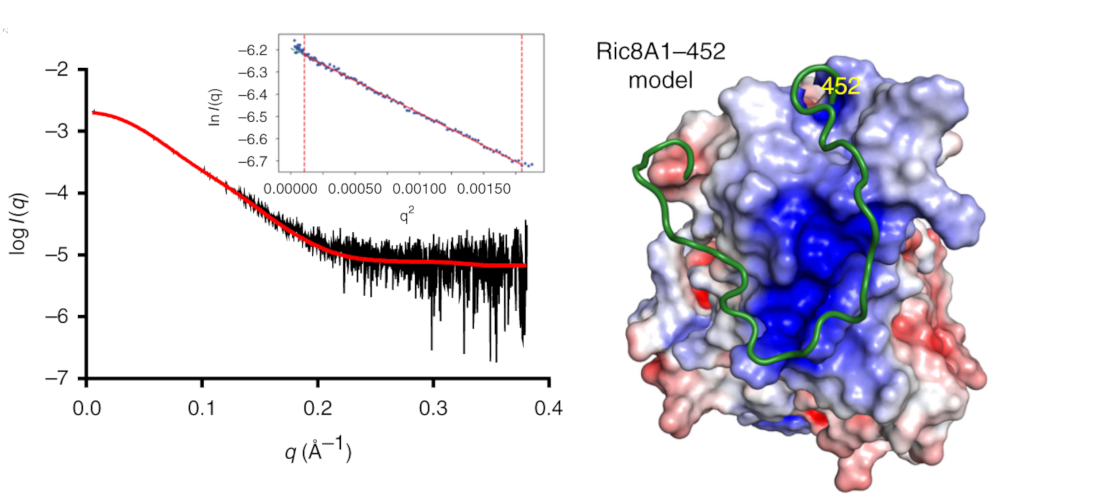
Structure of BS Ric8A, a regulator of G-protein Biology
Ric8A is a well-known regulator of G-protein biology and belongs to a class of proteins different from the G protein-coupled receptors (GPCRs), which act via interactions with monomeric Gα subunits as opposed to heterotrimeric Gαβγ proteins. SAXS was used in combination with crystallography and biochemical studies to show that the flexible C-terminal tail is important for the overall stability of Ric8A and the function as a guanine nucleotide exchange factor (GEF).
Learn More
Probing the Powering of Contractions in Heart Failure
Current treatments can slow progression of heart failure, but do not address the underlying issues, including specific problems that cause systolic heart failure. In this condition, the heart doesn’t contract vigorously enough in pushing blood into the body’s circulation. But findings at nanometer and millisecond scales, based upon experimental data collected at BioCAT may help improve design of therapies directed at motor proteins to rescue failing hearts.
Learn More
New Insights into Traumatic Brain Injury
Traumatic brain injury, or TBI, is often referred to as the “invisible injury” — while on the surface everything seems normal with brain structure, symptoms may present themselves in the behavior of the injured and cannot be explained. This work looked at the effect of controlled amounts of compressive force on rat optic nerves to attempt to identify the changes that occur in otherwise normal looking brain neurons due to the specific impact forces experienced during head trauma. As a result of this ongoing work, researchers have a better understanding of what kind of experience, or injury, leads to what kind of damage in the myelin - helping to visualize injuries based on the smallest force necessary to cause it. This information may be critical to knowing when someone has an injury after an accident but before symptoms emerge, and help supports the decision of when and how to treat them.
Learn More
Mechanistic Insights into Insulin Degrading Enzyme from Laminar-Flow SAXS
Insulin Degrading Enzyme (IDE) is known known to be a significant factor in the pathophysiology of conditions such as Diabetes mellitus and Alzheimer’s disease. This paper reveals structural states present during substrate recognition and capture and identifies a potential rate limiting step in the reaction.
Learn More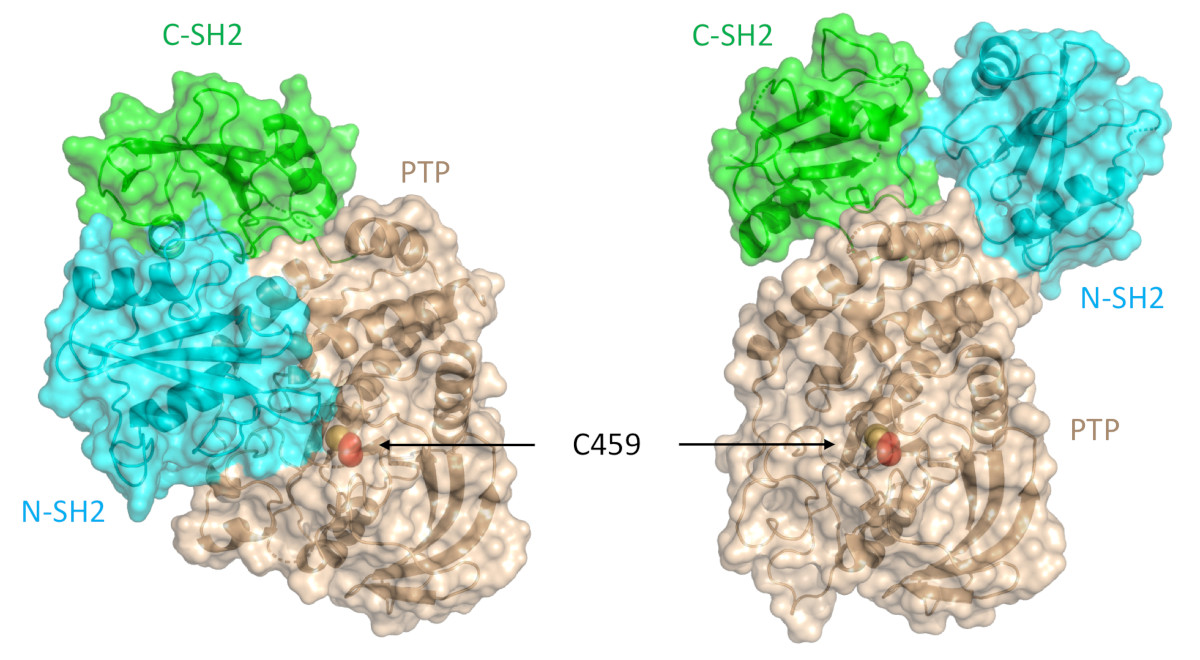
A Target Mutation that Renders a Cancer Drug Ineffective
Mutations in the gene PTPN11, which encodes a common enzyme called SHP2, can result in developmental disorders, such as Noonan Syndrome, and act as oncogenic drivers in patients with certain blood cancers. Due to the well understood role of the enzyme SHP2 in Noonan Syndrome and in tumorigenesis, many companies are currently trying to develop drugs that inhibit the enzyme. Researched investigated what impact mutations to SHP2 may have on the potential efficacy of drugs targeting this enzyme.
Learn More
A Super-relaxed Myosin State to Offset Hypertrophic Cardiomyopathy
Researchers investigated the stabilizing action of mavacamten, a cardiac drug currently in phase 3 clinical trials, on the ß-cardiac myosin super-relaxed state and its possible therapeutic effects on hypertrophic cardiomyopathy.
Learn More
Unraveling the role of a "nebulous" protein
Nebulin is a protein important to muscle strength, as mutations can cause the muscles in patients with nemaline myopathy disease to be weak, little is known about how it works. Researchers investigated the function of Nebulin in mice and found that it is necessary for generating physiological levels of force.
Learn More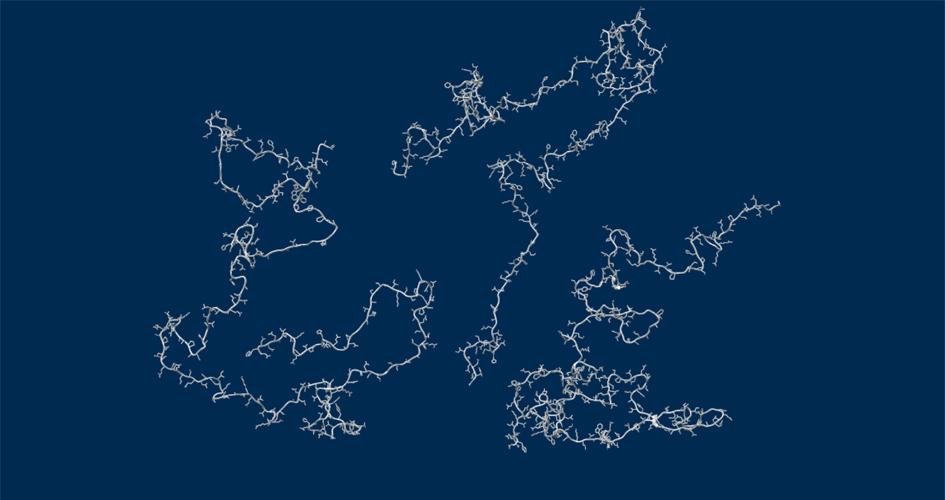
Proteins May Prevent Dysfunction and Disease by Relaxing
A new study suggests many proteins remain expanded in the cell, rather than contracting into tight folded shapes.
Learn More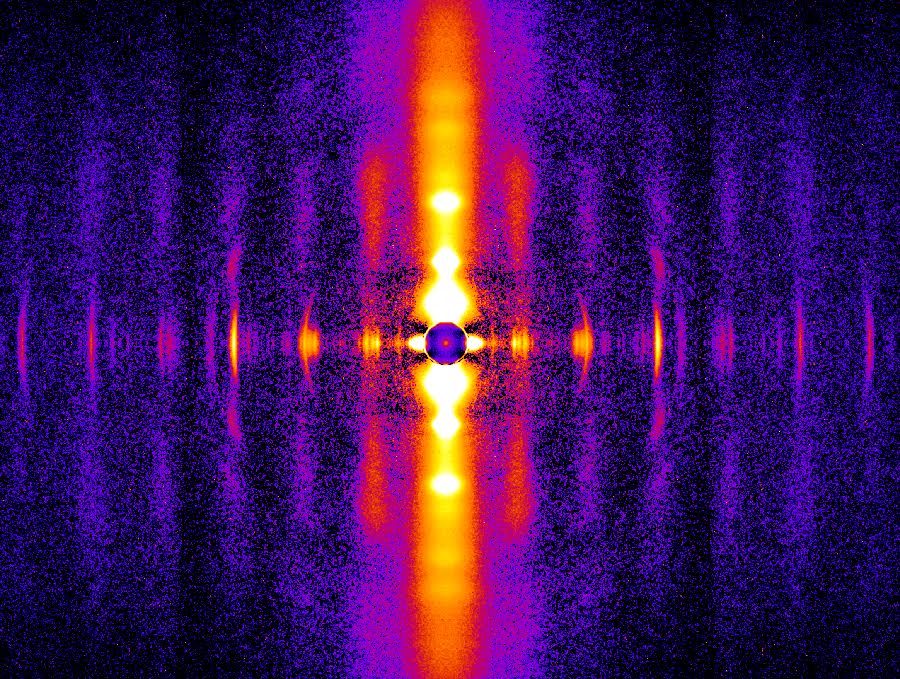
Unearthing the Mechanism of the Frank-Starling Law
Recent X-ray diffraction experiments show that the protein titin is critically important for transmitting the stretch-induced signals within the heart’s muscles known to impact the strength with which the heart contracts. This work not only solves a piece of the mystery of how the frank-Starling law determines cardiac function, but provides an avenue for targeted development of drugs to treat heart failure.
Learn More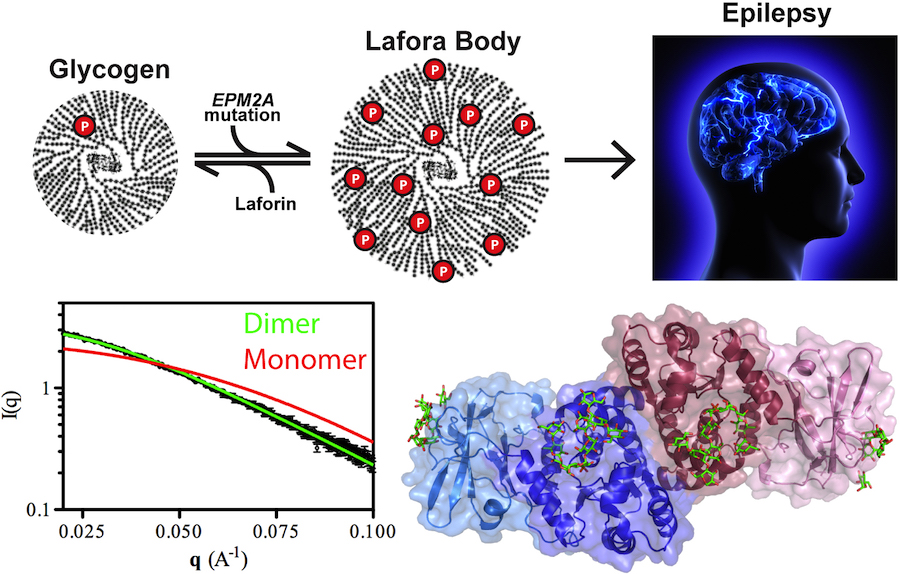
Lafora Disease: A Delicate Solubility Problem
Cells can store up to 55,000 glucose units in water-soluble spheres of branched, polymeric glycogen. This provides ready energy for rapid response to cellular needs but also must be managed carefully because too much glycogen accumulation can activate programmed cell death. This is especially true of neurons, which consume large amounts of glucose but are particularly sensitive to glycogen build-up. One example of what can happen when this basic metabolic process goes awry is observed in Lafora disease, a devastating fatal epilepsy in which mutations in a single key enzyme result in the formation of insoluble glucan inclusion bodies that cause neuronal death. Research conducted at two x-ray beamlines at the U.S. Department of Energy’s Advanced Photon Source (APS), an Office of Science user facility at Argonne solved the structure of the enzyme responsible, the laforin glucan phosphatase. The work has provided important insights into both the basis of Lafora disease and normal glycogen metabolism.
Learn More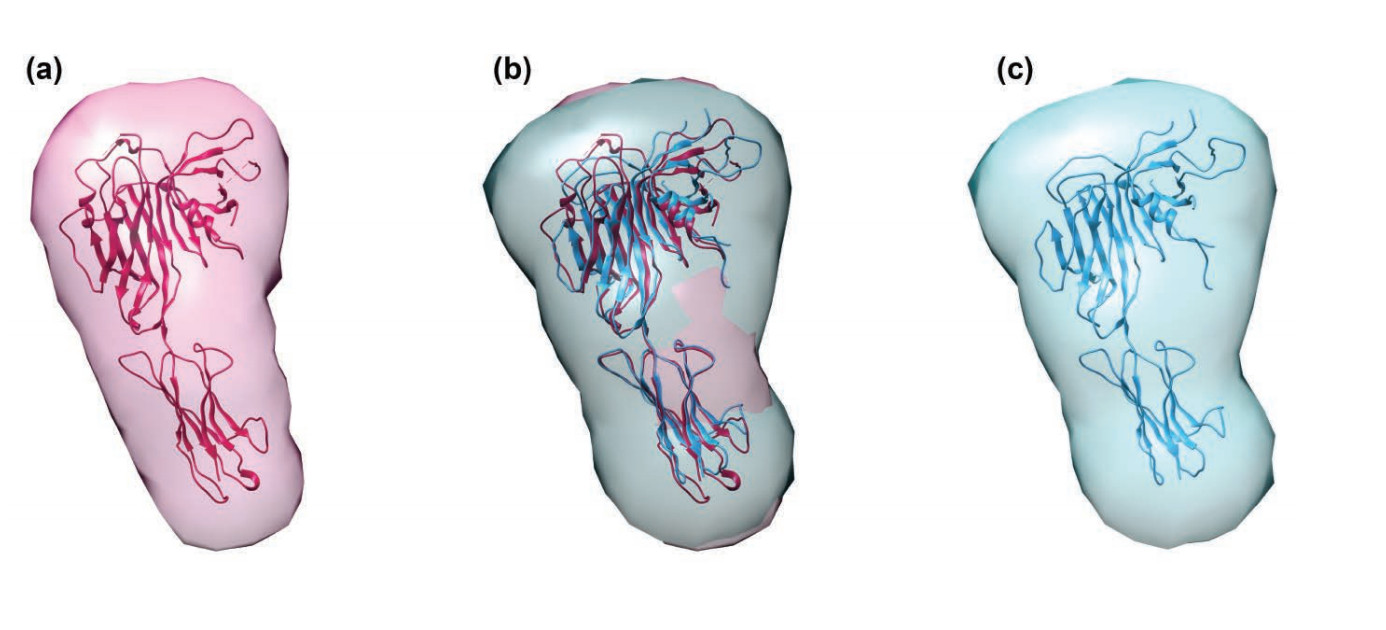
TAPBR: A Novel Protein Chaperone With a Role in Peptide Editing in Immune Recognition
TAP binding protein, related (TAPbPr), a novel protein chaperone, plays a role in loading peptides onto major histocompatibility class i (mhc i) molecules during the process of immune surveillance. Researchers investigated the biochemical function of TAPbPr, comparing it with tapasin, another chaperone with a similar protein sequence. The results of this study could lead to ways to modulate peptide loading in vaccine design, improving T-cell recognition.
Learn More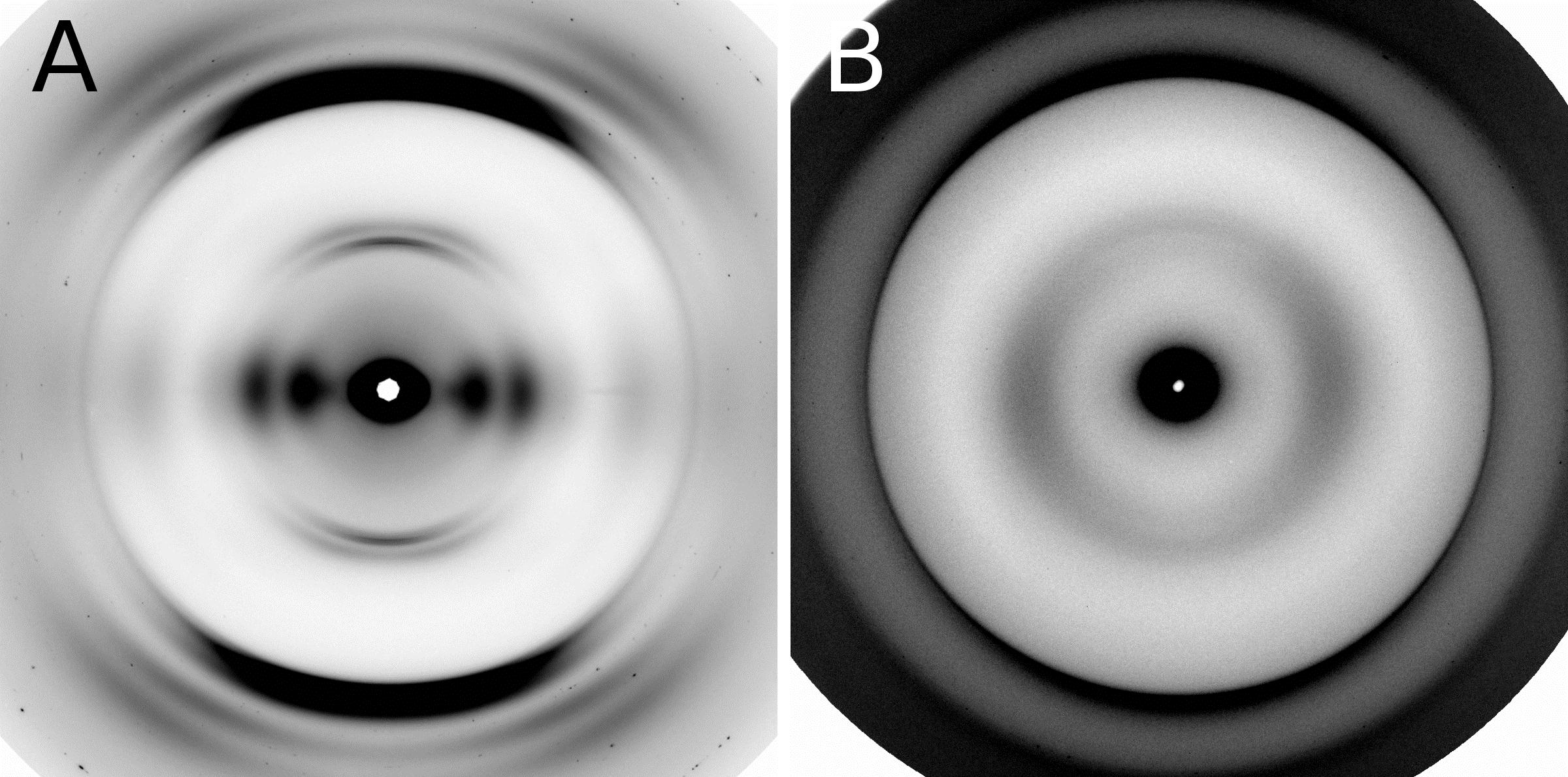
Combating an Infectious Invader
The investigation of the fungal prion HET-s(218-289) provides insights into the fundamental mechanisms of prion assembly and propagation of its infectious fold, which is made robust by a complex and diverse array of inter and intramolecular structural features. This level of complexity has not been observed in short-peptide amyloids that have been used as prion model systems.
Learn More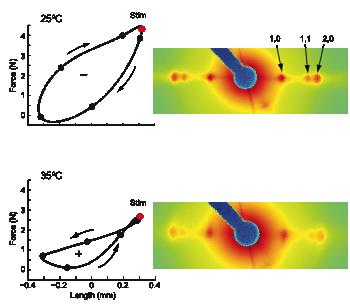
The cross-bridge spring: cool muscles store elastic energy
The Hawkmoth Manduca sexta is an emerging model system for a wide range of studies in integrative biology. The flight muscles are particularly interesting in that, unlike most insect flight muscle, but like vertebrate skeletal and cardiac muscles, they are a synchronous muscle where each stimulus generates one muscle twitch.
Learn More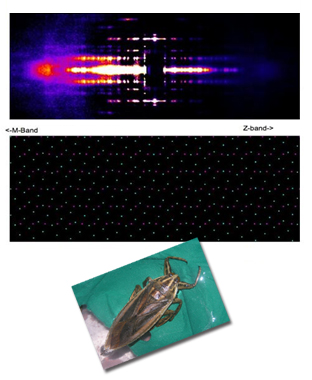
The Molecular Mechanism of Stretch Activation in Insect Muscle
Flying insects are among the most successful species on our planet. Flight is very metabolically demanding and many insects have found a clever way to reduce energy costs in their flight muscles by employing a process called “stretch activation, which has been recognized since the 1960s as an interesting and physiologically important phenomenon, but a mechanistic explanation has been elusive. Now, research at BioCAT provides another, important step toward a full explanation of stretch activation, which also plays an important role in mammalian cardiac expansion and contraction.
Learn More
Packing It In: A New Look at Collagen Fibers
Nature uses collagen everywhere in constructing multicellular animals. There are at least 20 types of collagen, but 80-90% of the collagen in the body consists of types I, II, and III. Collagen type II makes up 50% of all cartilage protein, and is essential in normal formation of such structures as cartilage, the vitreous humor of the eye (the clear gel that fills the space between the lens and the retina of the eyeball of humans and other vertebrates), bones, and teeth. To create these structures, collagen molecules are positioned in arrays called fibrils, producing what are known as the D-periodic fibrillar collagens. Until now, technical limitations prevented accurate structural studies of collagen type II packing. A research team aided by the BioCAT 18- ID beamline and the BioCARS 14-BM-C beamline at the APS has remedied that situation by determining the molecular structure of collagen type II in living tissues.
Learn More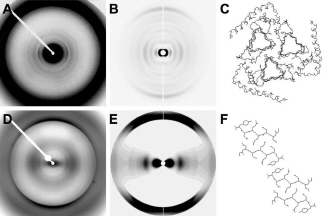
The Power of Proteins: Prion Diseases Demystified
It is hard to believe that a single protein can be responsible for the damage inflicted by diseases such as human Creutzfeldt-Jakob and bovine spongiform encephalopathy (Mad Cow Disease). Yet the implicated protein, known as a prion and only about 200 amino acids long, can initiate and propagate a disease cycle just by changing its shape. A collaborative research team has achieved a significant advance in our understanding of the infectious power of the prion protein.
Learn More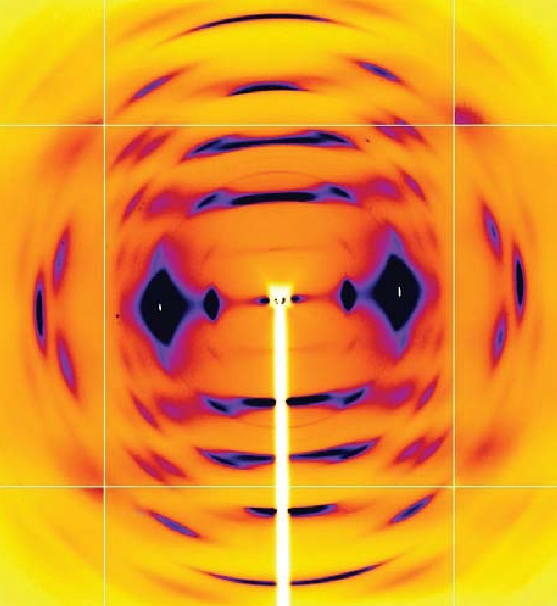
Getting to Know Cellulose
As humans continue to deplete the Earth’s supply of fossil fuels, finding new sources of energy becomes a priority. Biomass, such as cornhusks left after harvest, is one such alternative energy source. Before efficient use can be made of such materials, understanding how to break down cellulose—the fiber in human nutrition and the main component of much biomass waste—is crucial. With the help of the NE-CAT and BioCAT beamlines at the APS and the SPring-8 (Japan) beamline BL38B1, an international research team from Los Alamos National Laboratory, the University of Tokyo, and the University of Grenoble has identified important new features of cellulose structure. Their work provides important new details that could be used in designing more efficient treatments for cellulosic biomass.
Learn More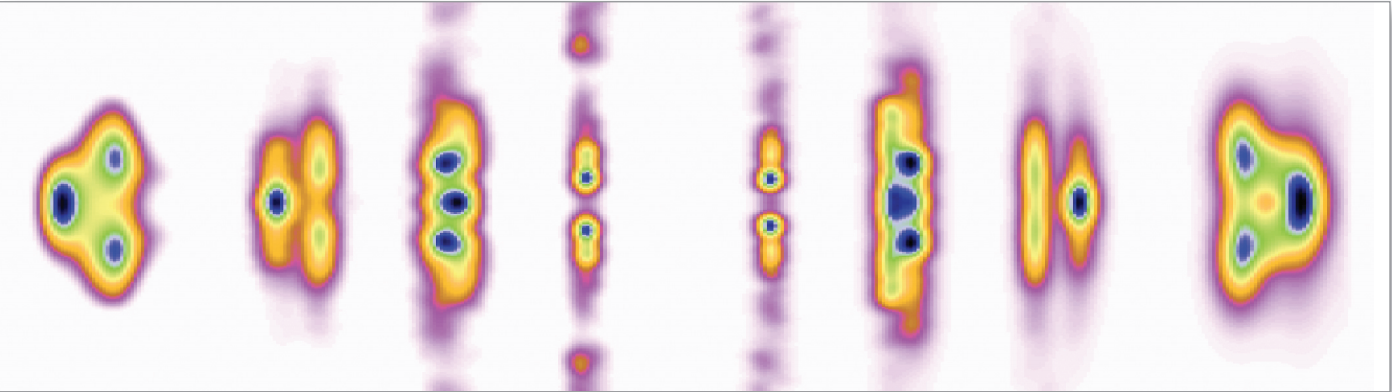
Filling the Gaps in Collagen Structure
Collagens—we might take them for granted, but without them there would be no way to build tissues of the heart, skin, cornea, or bones. In much the same way that wood is used to frame a house and form a structure for the overlying construction materials, collagens are proteins used in the framing of mammalian tissues, but gaining an accurate picture of their three-dimensional structure in the body has proven more difficult. Thanks to work by a research group based at the Illinois Institute of Technology and using the BioCAT 18-ID beamline at the APS, a complete structure for a collagen molecule—as it actually appears in the extracellular matrix (ECM)—is now available.
Learn More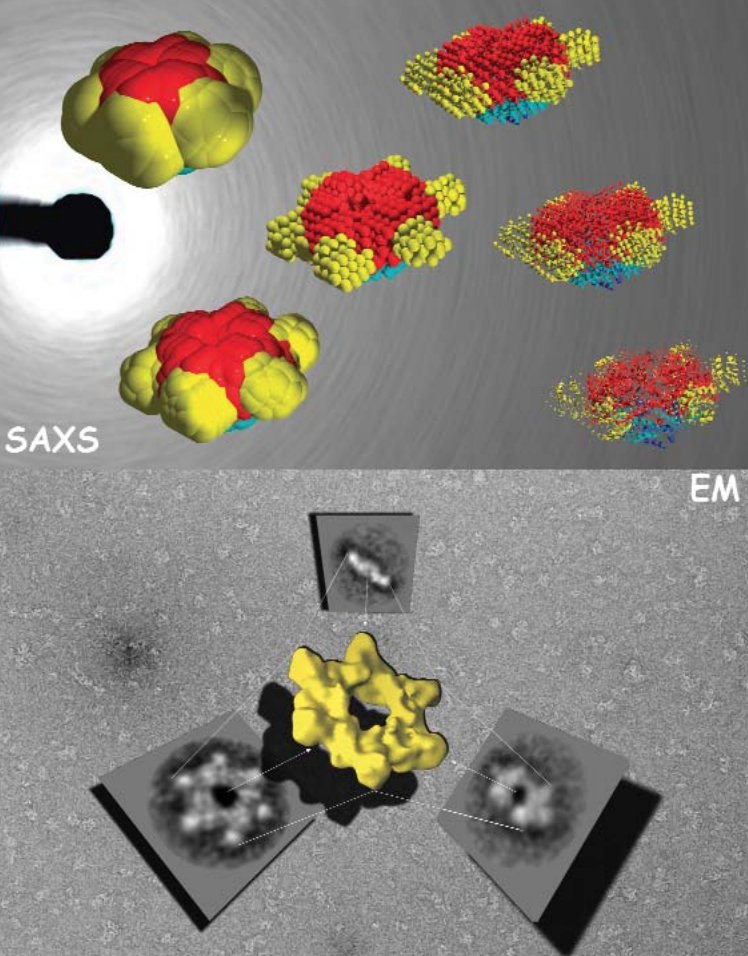
The Correct Signals to Regulate Assembly in Bacteria
By employing x-ray scattering and electron microscopy researchers using the BioCAT beamline were able to describe —in stunning detail— a novel two-component mechanism for assembling a protein associated with bacterial transcription. Their work greatly advances our understanding of what happens in normal and, by inference, diseased cells.
Learn More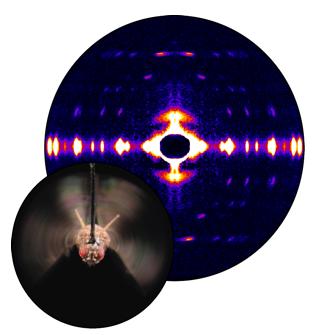
Storing the Power to Fly
Fruit flies beat their wings faster than their cellular powerplants can generate the energy needed for flapping. To resolve this energetic discrepancy, researchers used the BioCAT beamline to obtain a series of x-ray photographs that revealed the flies’ secret: A muscle protein used to power wings acts like a spring, storing energy while stretched before snapping back. Not only did this finding surprise researchers who study muscle, but the results might also help scientists better understand the human heart.
Learn More